COVID-19 Vaccine FAQs for Cancer Patients
The COVID-19 pandemic continues to have a serious impact on everyone, including cancer patients, their families and caregivers. In addition to existing actions, we know can help prevent spread of the virus (hand washing, wearing a mask, social/physical distancing, sanitizing surfaces regularly and staying home when sick or told to quarantine) there are now COVID-19 vaccines available to the general public.
People with cancer have a higher risk of getting seriously ill, being hospitalized, and dying from COVID-19. This is why it’s so important that people with cancer get vaccinated against the virus.
COVID-19 Vaccine FAQs for Cancer Patients
Every cancer patient’s situation is different, so we encourage you to discuss the risks and benefits of getting a COVID-19 vaccine with your oncologist.
Should people with cancer and undergoing active treatment be vaccinated against COVID-19?
The existing COVID-19 vaccines have been shown to be safe and effective for the general population and there is no evidence that they will not be safe for most cancer patients, as long as components of that vaccine are not contraindicated. Contraindications include anyone with a history of immediate allergic reaction of any severity to any part of mRNA COVID-19 vaccines or to polysorbate. People developing severe (such as anaphylaxis) or immediate allergic reactions after a first dose of mRNA COVID-19 vaccine should not receive a second dose.
It is possible that cancer patients could have a reduced immune response, which means the vaccine might not be as effective in protecting cancer patients from the effects of the COVID-19 virus as it would be for generally healthy individuals. For this reason, it is important that cancer patients continue to follow all current guidance (hand washing, wearing a mask, social/physical distancing, sanitizing surfaces regularly and staying home when sick or told to quarantine) to protect themselves against COVID-19.
Despite the possibility of a reduced immune response from the COVID-19 vaccine, it may still offer some benefit and is important to reduce the risk or severity of COVID-19 to cancer patients.
The only exception to this is for people in the process of receiving a stem cell transplant or CAR T-cell therapy. If you are currently receiving a stem cell transplant or CAR T-cell therapy, you should wait at least three months after treatment to get vaccinated. UT Medical Center’s Transplant and Cellular Therapy team requires all patients and donors who are accepted into the Transplant and Cellular Therapy program to be fully COVID-19 vaccinated prior to starting their transplant or cellular therapy process.
If you have a history of immediate allergic reaction to other vaccines or injectable medications, you should discuss with your physician the risks of developing a severe allergic reaction and balance these risks against the benefits of COVID-19 vaccination.
Timing matters for patients who choose to get vaccinated.
Cancer patients currently receiving treatment for cancer should talk with their treatment team about the best plan for the timing of their vaccination.
Oncologists have experience providing other types of vaccines to patients receiving treatment for cancer. While there are no safety concerns about vaccination for patients who are in treatment, the COVID-19 vaccines will be more effective if timed in coordination with your cancer treatment schedule. Plans such as providing the vaccine in between cycles of therapy and after appropriate waiting periods for patients receiving certain treatments can be used to reduce the risks while maintaining the success of vaccination.
Should cancer patients and cancer survivors receive additional doses of the COVID-19 vaccination?
YES, if you have active cancer or if you’re immunocompromised. Moderately or severely immunocompromised people may not mount a protective immune response after initial vaccination and, furthermore, their protection by primary vaccination may decrease over time.
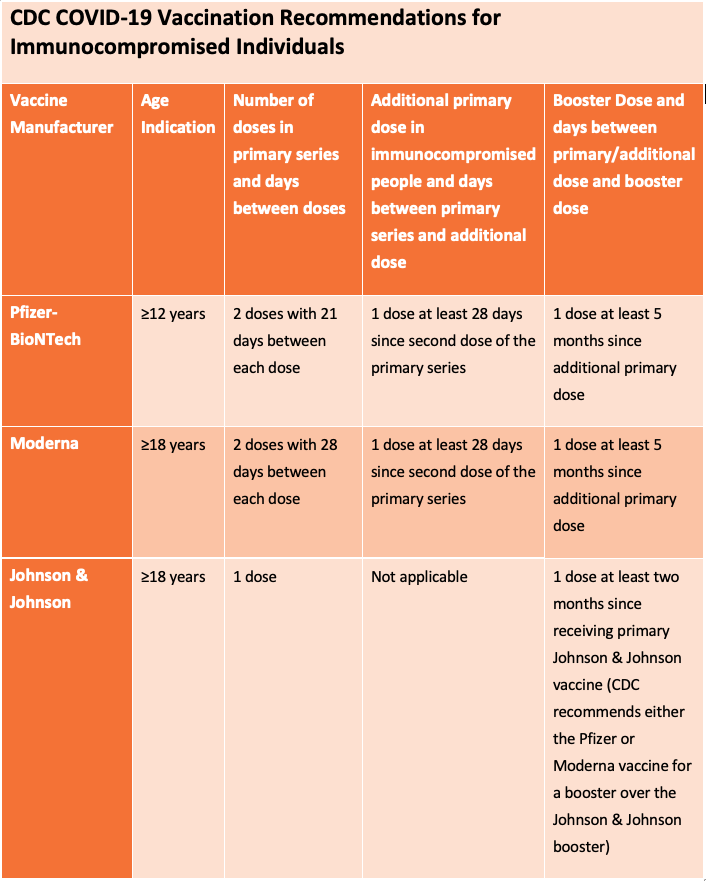
The Centers for Disease Control and Prevention (CDC) recommends an additional primary dose of either the Pfizer/BioNTech or Moderna vaccine for people who are moderately or severely immunocompromised at least 28 days after completion of the initial 2-dose mRNA COVID-19 series, as well as a booster dose at least five months after completing their additional primary dose. If a moderately or severely immunocompromised person aged 12 years or older has received two primary mRNA vaccine doses but has not yet received an additional mRNA primary dose, they should first receive the additional age-appropriate primary dose (at least 28 days after the second dose), followed by a single age-appropriate COVID-19 vaccine booster dose (at least 5 months after the additional primary dose).

The CDC recommends that the additional primary dose be the same mRNA COVID-19 vaccine as was administered for the primary series. For example, if you received the Pfizer/BioNTech for your first two primary doses, your additional primary dose and booster should also be Pfizer/BioNTech.
The Johnson & Johnson COVID-19 vaccine is not authorized for use as an additional primary dose and people who received a single-dose Johnson & Johnson primary vaccine should not receive an additional primary dose. You can however receive a booster dose of either mRNA COVID-19 vaccine (Pfizer or Moderna) two or more months after receiving the one dose Johnson & Johnson COVID-19 vaccine.
NCCN experts recommend additional vaccine doses for patients with cancer or those who are immunocompromised, especially for:
- People who are currently being treated for tumors or blood cancer or who received cancer therapy within 1 year of their 1st dose of the COVID-19 vaccine
- People with newly diagnosed or recurrent cancer who will receive cancer therapy
- People with hematologic cancers (eg, chronic lymphocytic leukemia, multiple myeloma, myelodysplastic syndrome, or chronic myeloproliferative neoplasms) whether they’re receiving cancer therapy or not
- People who had an organ or stem cell transplant, cellular therapy (CAR T cells) or take medicine to suppress your immune system for another condition
- People with cancer and other conditions that suppress the immune system (eg, HIV infection or taking steroids or other drugs that impair the immune system)
Should family living with immunocompromised individuals get a booster dose?
The NCCN does recommend that people living in the same household with immunocompromised individuals should also get a booster dose once it is available to them.
Where should I go to receive the vaccine?
UT Medical Center is offering the COVID-19 vaccine (all three doses) in our vaccination clinic. Click here to schedule an appointment.
If you are unable to book an appointment with us, please visit www.vaccine.gov to find the nearest location that is offering COVID-19 vaccinations.
Should cancer survivors be vaccinated against COVID-19?
Cancer survivors may be offered vaccination against COVID-19 as long as any components of the vaccine are not contraindicated.
Are there people who should not be vaccinated?
At this time, only those with contraindications (outlined above) to a specific vaccine component should not be offered vaccination with that specific product.
Please discuss with your oncologist any concern you might have about a potential contraindication to the vaccine.
What other concerns are there for people with cancer who are vaccinated?
As there is still uncertainty about how much protection the COVID-19 vaccine will provide immunocompromised patients with cancer, vaccinated patients and their caregivers should continue to follow current guidance (hand washing, wearing a mask, social/physical distancing, sanitizing surfaces regularly and staying home when sick or told to quarantine) to protect themselves from exposure to COVID-19.
Is the COVID-19 vaccine free if I am eligible to receive it?
Yes, the COVID-19 vaccine will be free to anyone who wishes to get one. However, some vaccination providers may charge an administration fee for giving the shot.
Are there side effects associated with receiving the COVID-19 vaccine?
No serious side effects have been seen. However, some people have experienced headache, fever, body aches, felt tired or had some redness and injection site discomfort. These symptoms usually go away within a couple of days.
Sources
Contact Info
Cancer Institute1926 Alcoa Highway
Medical Building F
Knoxville, Tennessee 37920
865-305-6055
1-866-337-8824
Find a Doctor
Contact Info
Cancer Institute1926 Alcoa Highway
Medical Building F
Knoxville, Tennessee 37920
865-305-6055
1-866-337-8824
Find a Doctor
